The Collection of Minerals
in the
American Museum of Natural History
By Herbert P. Whitlock
Curator of Mineralogy
Guide Leaflet No. 49
Third, Revised Edition
New York, June, 1926
The Morgan Memorial Hall of Minerals and Gems
How to Make Use of the Collections
The collections displayed in this Hall represent the minerals that have been found throughout the world, They come from the earth's crust, which is entirely made of them. Some of them have been found on the actual surface where the rocks are exposed to view; others were taken from mines, quarries and other excavations.
In order to understand what minerals are and what they are made of, consult the first case on the right under the heading, "What is a Mineral."
To become familiar with the characteristics of minerals, by which they may be distinguished from one another, consult the Introductory Series explaining the outward form, color, luster, etc., of minerals in the four cases to the right of the entrance.
A brief summary of the collection may be obtained from the Wall Cases beginning on the left of the entrance and continuing around the walls.
The Main Collection of Minerals is displayed in the cases to the left and right of the entrance, beginning on the extreme left and proceeding from left to right, as the page of a book is read.
The Collection of Gem Stones, showing the adaptation of minerals to gems and ornaments occupies the series of cases extending down the middle of the Hall. Begin with the case on the right, proceed from left to right along the north row, returning along the south row.
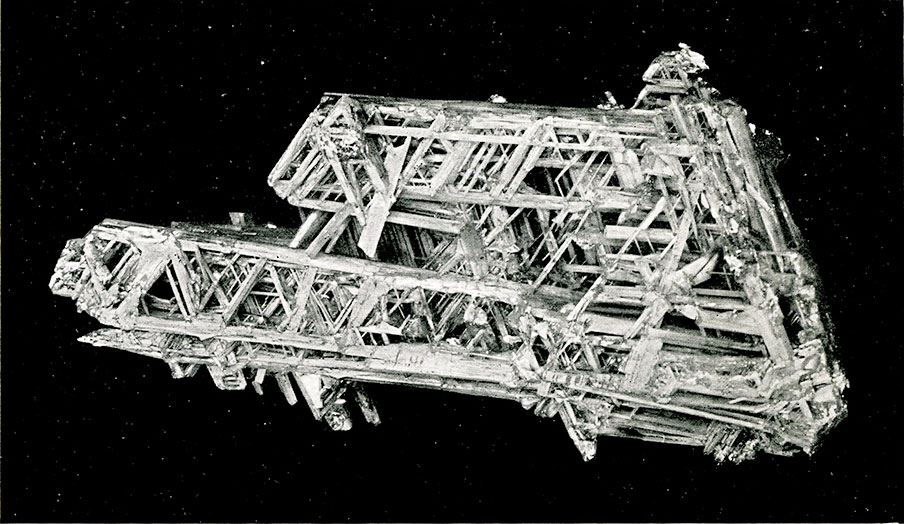
Frontispiece. Cerussite from Broken Hill, New South Wales. A net work of delicate, interlaced crystals.
The Collection of Minerals
Introduction
Below the very thin layer of vegetable matter, the function of which is to support life, the mass of our globe, as far as our knowledge of it extends, is composed of a number of inorganic substances which are known as minerals. These singly or in aggregates of two or more make up the rocks which in many places are a conspicuous part of the scenery, and important building material. They furnish us with the raw material from which we derive the metals so useful to us in the arts, and even in their decay they provide many of the soil components necessary to vegetation, But essential as these economic minerals just alluded to are, they form a comparatively small part of the great array of natural compounds which come under the classification of minerals. Every substance to be found upon this earth, which has not been directly formed from animal or plant life, and many which come to us in the form of meteorites from outside the earth's atmosphere, are included in the mineral kingdom. There are over 1100 different kinds of minerals known, and the list is constantly being added to as new mineral substances are being found in mines and quarries in every part of the globe. Many of those are very rare, and have only been discovered in one or two places, but some of them, such as quartz, calcite and the feldspars, are widely distributed and common enough to be familiar to almost every one. Most of the known mineral species are to be found in the collection to which this Guide Leaflet serves as an introduction, and inasmuch as many of them to the casual eye appear very much the same, a word or two is necessary to enable the visitor to single out some of the characteristics which serve to distinguish them. Although in many instances a mineral, such as for example sulphur, has a characteristic color, it is not difficult to find among the many other species and varieties of minerals one which has almost if not quite the same tint. Color, then, is far from an infallible means of identifying a mineral. Many of the metallic minerals have what is known as a metallic luster, such as the yellow brass-like sheen of pyrite or the black steel gray glint of stibnite. But even this is not an unvarying mark of distinction, for galena, the lead sulphide, has a color and luster almost identical to stibnite, the antimony sulphide; and many of the ores of the metals, such as smithsonite, the carbonate of zinc, and malachite, the carbonate of copper, show a luster which is not at all metallic. Minerals do, however, possess a property which is very useful in identifying them. With very few exceptions every mineral species has a more or less pronounced tendency to form in solids with regular outlines, smooth bright faces and sharp angles. These solids which are called crystals are distinctive, each mineral having its characteristic series of forms, some occurring in cubes, others in slender needle-like prisms, and others in flat angular plates. Although the very great diversity and intricacy of these crystal forms of minerals are somewhat bewildering to anyone unfamiliar with this highly fascinating branch of science, one soon finds that they are capable of being divided into a small number of very simple groups. A series of models showing some of the more important forms of crystals and their relation and meaning will be found to the right of the entrance to the Mineral Hall.
The History of the Collection
Like most of the large mineral collections of the world, the collection displayed in the Morgan Hall of Minerals has been the result of slow growth over a considerable period. The nucleus of the present collection was the Bailey Collection, a relatively small series of the commoner minerals, but one which was thoroughly comprehensive and served well in the early days of the Museum to represent this branch of Science. The first large addition came in 1891, when the Spang Collection was purchased and not only more than doubled the number of specimens in the Museum but added many new species to those already displayed. It was in 1900, however, that the Collection took rank as the most complete as well as the richest in notable specimens in America and one of the five best exhibition collections in the world. Through the gift of the late J. Pierpont Morgan, Esq., the Museum acquired the remarkable collection of minerals brought together by Mr. Clarence S. Bement of Philadelphia. This last addition, which comprises a large percentage of the specimens now displayed in the Morgan Hall of Minerals, is famous for the exceptional perfection of the material comprising it. The quality of this material, both from the point of view of its scientific interest and the size and beauty of its examples, may be best understood when one considers the fact that Mr. Bement, a collector of rare judgment and appreciation, not infrequently purchased an entire small collection in order to acquire a single specimen of unique value. Since the gift of the Bement Collection many additions of exceptional beauty and interest have been acquired by purchase from the Fund established in 1904 by Matilda W. Bruce. The Mineral Collection which thus attained a high standard of merit has been augmented year by year through careful selection of the best available specimens of the more recently discovered species, varieties and occurrences. The more newly acquired of these will be found in the small cases of Recent Accessions displayed near the center of the Hall. In 1922 the Mineral Hall was completely remodeled architecturally through the generosity of Mr. George F. Baker who chose this means of honoring the memory of his friend and associate the late John Pierpont Morgan. Thus the Hall is now designated as the Morgan Memorial Hall of Minerals and Gems. Marble tablets set in the middle of the south wall and between the middle windows of the north wall commemorate respectively this presentation, and the names of the donors of important Mineral and Gem specimens.
Classification of Minerals
A mineral is a natural chemical compound, that is, it has in most instances a definite chemical composition, and it is this chemical composition, constituting as it does the essential and unvarying characteristic of a mineral, which forms the basis of its classification. There are many thousands of compounds known to chemists which include the 1100 or more natural compounds, or minerals. But all of these when reduced to their simplest constituents are proved to be made up from combinations of a relatively small group of ultimate substances called elements. Of the 80 or more elements at present known, there are 20 which are so common that they make up 99½ percent of the surface layer of the earth's crust to a depth of 10 miles which marks the limit of our knowledge, and of these 20 only 8 are needed to constitute 97 percent of this surface layer. The 20 commonest elements in the order of their abundance are:
| 1. | Oxygen | O | 5. | Calcium | Ca |
| 2. | Silicon | Si | 6. | Potassium | K |
| 3. | Aluminum | Al | 7. | Sodium | Na |
| 4. | Iron | Fe | 8. | Magnesium | Mg |
| These constitute 97 per cent | |||||
| 9. | Titanium | Ti | 15. | Manganese | Mn |
| 10. | Hydrogen | H | 16. | Chlorine | Cl |
| 11. | Carbon | C | 17. | Strontium | Sr |
| 12. | Phosphorus | P | 18. | Fluorine | Fl |
| 13. | Sulphur | S | 19. | Zirconium | Zr |
| 14. | Barium | Ba | 20. | Nickel | Ni |
In order to understand better the chemical system used as a basis for classifying minerals, it is more convenient to group these 20 common elements into two classes, metals and non-metals.
Metals. Aluminum, Iron, Calcium, Potassium, Sodium, Magnesium, Titanium, Barium, Manganese, Strontium, Zirconium, and Nickel.
Non-metals. Oxygen, Silicon, Hydrogen, Carbon, Phosphorus, Sulphur, Chlorine and Fluorine.
It is this last series of the non-metals which is especially important to remember, because in the combinations of one or more non-metals with one or more metals which, in general, go to form minerals it is the non-metals which determine in what class the mineral is to be placed. So we have for some of the principal divisions of the classification of minerals.
Sulphides, composed of sulphur and some one or more of the metals, as sulphide of copper, the mineral Chalcocite.
Chlorides, composed of chlorine and a metal, as chloride of sodium, the mineral Halite.
Oxides, composed of oxygen combined with some of the metals, as oxide of iron, Hematite.
The oxides of the metals, which have different properties from the uncombined metals, sometimes combine with the oxides of the nonmetals and form more complex compounds which are called oxygen salts and constitute important divisions of the mineral classification. Some of these are the Carbonates, the Silicates, the Phosphates, the Sulphates, etc.
Names of Minerals
It is a general rule in the natural sciences, such as Botany and Zoology, to preserve in the name of a plant or animal either some word of Latin or Greek origin (because these are at present the universal languages of science) which describes a characteristic of the species or to perpetuate in naming it the surname of some distinguished man connected with its discovery. This very general rule has been applied to the naming of minerals and the termination ite or lite (originally from λίθος, a stone) is almost always added. For example, Hematite is named from the Greek word for blood because its common varieties are red in color; Haüynite is named after the French crystallographer Haüy, and Andalusite is named from the ancient province Andalusia, in the South of Spain, where it was first found. This last name is an example of the practice of naming some minerals after the place where they were discovered. Some mineral names which do not end in ite are survivals of a time when the science was in its infancy and recognized few species. Many of these as Quartz, Garnet, Gypsum, Corundum and Spinel, are so old and well established that they have come down to us unchanged.
Guide to the Collection
The collection of minerals displayed in the Morgan Hall of Minerals is without question one of the finest to be found in the world. Although remarkably complete in its representation of most of the mineral species known to science this collection is especially noteworthy for its assemblage of splendid examples of the commoner and more widely distributed minerals.
The visitor should begin with the first of the upright pier and table cases to the left of the entrance and proceed from left to right along each side of every case throughout the series, advancing from east to west along the south side, crossing to the north side at the west end of the hall and following the numbering of the cases back to the east entrance. Each case is furnished with a descriptive label referring to its contents and indicating the wall case of the series, arranged along the east, south and north walls, which contains large and handsome specimens of the same species. These latter are placed in close proximity to the corresponding table cases of the principal series and can be readily located.
Sulphur from Girgenti, Sicily. Clear, well developed crystals.
Stibnite from Ichinokawa Mine, Iyo, Japan. A group of slender prismatic crystals.
Elements
Cases 1, A and B
This small but important division of the mineral classification includes those elements which occur in nature uncombined, or in a "native" state, as native gold and native bismuth. Chemically they are the simplest of all minerals and consequently the best with which to begin the inspection of a series which increases in chemical complexity as it develops.
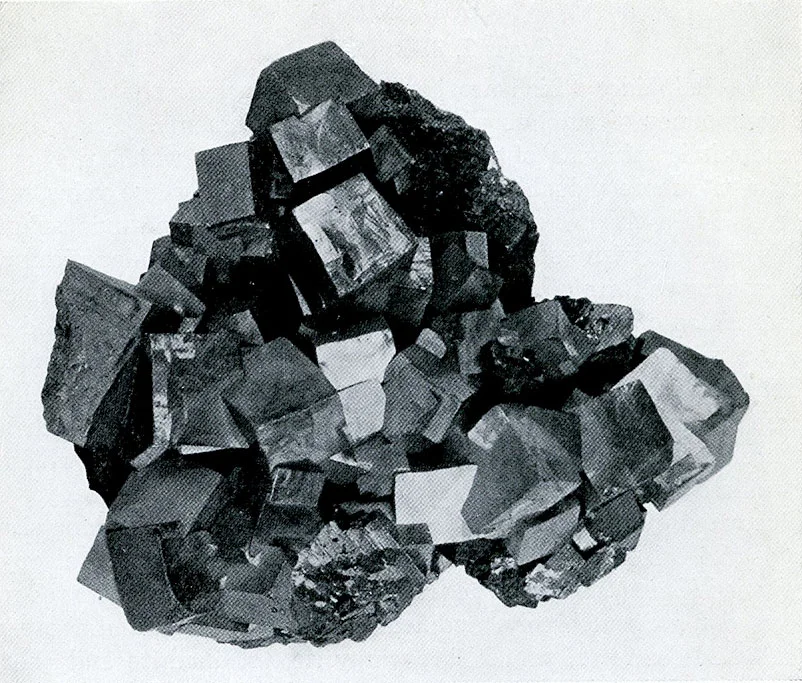
Galena from Galena, Ill. Clearly defined cubic crystals which were deposited on the walls of an open vein, the galena being the last mineral to form in the vein.
Two kinds of native carbon, diamond and graphite, will be found in Case 1. These are widely different in appearance and properties and illustrate the way in which, under different conditions of formation, the same chemical substance may yield dissimilar modifications. The beautiful groups of yellow sulphur crystals furnish the first glimpse of the wonderful intricacy and symmetry of the crystal forms of minerals (compare with model in Case 25). Sulphur is formed near active or extinct volcanoes and in the beds of gypsum, where it constitutes a decomposition product. The native metals are represented by gold in nuggets, veins and crystal aggregates, silver in wire-like and branching forms, and copper in beautifully developed crystals and crystal masses, presenting a great variety of shapes. All these are readily recognizable in luster and color from our association of them with coins, jewelry and other familiar things.
Sulphides
Cases 2, 3, B, C, D and F
The Sulphides which are here made to include the Sulpho-Salts are compounds of sulphur with the metallic elements. They are the characteristic minerals of the metallic veins from which the greater part of the more valuable metals are derived. In these veins, which were originally fissures or clefts in the rocks, vapors and fluid solutions highly charged with sulphur and with dissolved metals deposited their contents in the form of sulphides. The openings in this way ultimately became filled or partly filled with these minerals, which are called ores, together with associated unproductive minerals, such as quartz, calcite, and fluorite, which are known as gangue minerals. The finest and most characteristic specimens come from the parts of the veins which have not been completely filled in the process of formation and in which the crystallized minerals have had a chance to separate individually. This is illustrated by the handsome groups of stibnite crystals in long slender prisms (Case C), the varied series of galena, sphalerite and chalcopyrite specimens (Cases 2 and 3), the wide range of pyrite specimens, showing many complex and highly modified crystals (Case 3), and the exceptionally fine series of proustite pyrargyrite, tetrahedrite and enargite (Cases 3 and 4). This division also includes many rarer minerals in notable specimens, such as ullmannite, sylvanite, emplectite, binnite, cosalite, bournonite, jordanite and stephanite.
Haloids
Cases 4 and G
This division of minerals includes the compounds of the metals with elements of the chlorine groups, the latter being known as halogen elements and comprising chlorine, bromine, iodine and fluorine. These give the chemical compounds called chlorides, bromides, iodides and fluorides.
Marcasite from Felosbanya, Rumania. A radiated aggregate of flat crystals.
Some of the haloids, as exemplified by the mineral halite or rock salt, the chloride of sodium, occur in nature in extensive beds and have been deposited by evaporation from bodies of water which have in times past been cut off from the main body of the ocean. The series of halite specimens in Case 4 includes many striking examples of large and well-developed crystals.
Fluorite from Cumberland, England. Encrusted with quartz on the edges and corners of the cubic crystals
The most widely distributed mineral in this division is fluorite, the fluoride of calcium. This is essentially a vein mineral and is frequently found associated with the sulphide ores of lead and zinc. The large cubic and octahedral crystals of fluorite from all parts of the world shown in Cases 4 and G, illustrate the very great variation in color which is a characteristic of this mineral and which is due to the presence of a slight amount of such impurities as iron and manganese. Many of the fluorite specimens show bandings of color produced by slight changes in the composition of the mineral-forming solution. Among the oxychlorides in Case 5 will be found some beautiful examples of the rare copper minerals boleite and percylite.
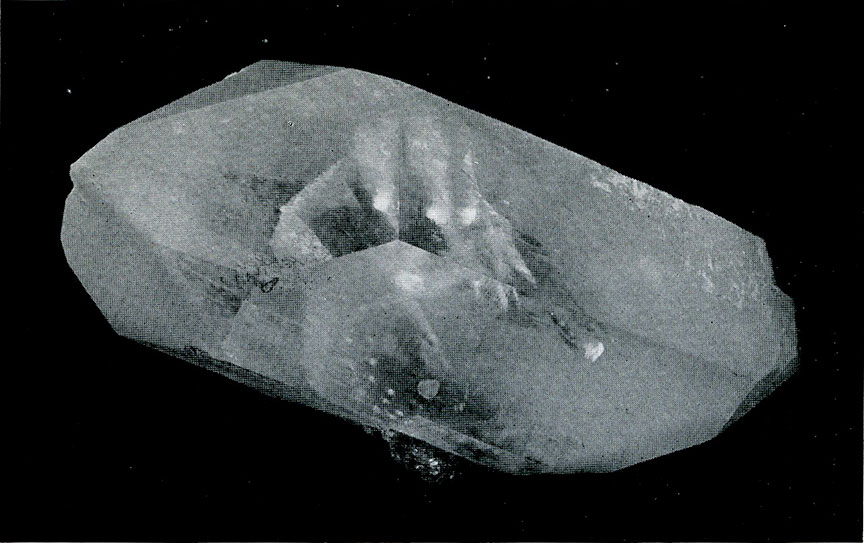
Quartz from California. A "phantom" showing one quartz crystal deposited around one previously formed.
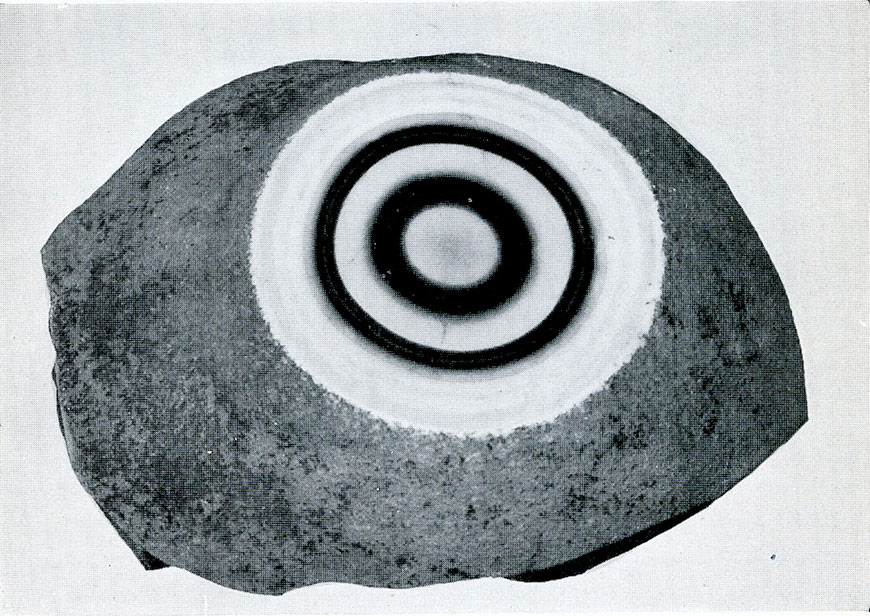
Quartz from Uruguay, S. America. Agate formed of layers of differently colored quartz.
Oxides
Cases 5–8 and H–N
Oxygen, one of the most energetic of the elements, also constitutes a large part of the atmosphere of the earth, and of water which is almost universally present on it. Consequently, as we would expect, the oxides, or compounds of oxygen with the metals, form a large and important division of minerals. But the element silicon ranks next to oxygen in abundance and combines readily with it. It is therefore quite obvious that the mineral quartz, which is the oxide of silicon, should be the commonest and most widely distributed of all minerals.
Hematite from St. Gothard, Switzerland. A rosette of flat crystals or "iron rose."
The suite of quartz specimens beginning in Case 5 is exceptionally fine. At the head of the series will be found the sharply defined, brilliant, transparent crystals which are familiar to most of us. These are characteristically six-sided with prisms of varying length and occur as single crystals or in groups. In addition to the specimens showing the wide range of crystal habit, attention is particularly directed to the examples of phantom quartz and capped quartz, which illustrate the effect of a change in, or the temporary arresting of the action of the silica-depositing solution, the result in both cases being the production of a quartz crystal around a similar and previously formed one. Small amounts of such impurities as titanium, manganese and organic matter produce respectively the colored varieties, amethyst, rose quartz and smoky quartz (Cases I and K).
There are also many examples of quartz enclosing other minerals, such as sagenite, enclosing slender needles of rutile, and cat's-eye quartz, in which the silica solution has surrounded and imprisoned hair-like fibres of asbestos. The massive forms of quartz (Case 6) are distinguished by their entire lack of outward evidences of crystallization. Here the mineral assumes rounded outlines, similar to and produced in much the same way as the icicles of frozen water or the stalactitic deposits which are formed by the dripping of mineral solutions in a cavern. Beginning with chalcedony, which well illustrates the deposit of quartz from a silica solution of uniform composition, the series shows a great variety of agates, in which the layers of differently colored quartz have been produced by a change in the amount and character of the coloring impurity in the silica-depositing fluid. Considerable amounts of iron and clay give rise to the opaque, massive varieties, jasper and basanite.
Opal is a hydrated oxide of silicon, that is, it has the same chemical composition as quartz except that it contains a varying percentage of water. Among the many varieties of opal in Case 6 the one which appeals most strongly on account of its beauty is precious opal. The brilliant and varied play of color which is a well-known characteristic of this mineral is supposed to be caused by incipient cracks in the mass of the stone. These reflect back the light in the same way as the film of a soap bubble or of oil spread on water. Both massive quartz and opal under favorable conditions replace the woody tissue of trees, producing jasperized wood and opalized wood. These varieties, when polished, exhibit very strikingly the outlines of the cellular structure of the wood which has thus become petrified.
Rutile from Parkesburg, Pa. Rosettes of knee-jointed crystals.
Maganite from Ilefeld, Harz, Germany. Bundles of closely grouped crystals.
Limonite from Rossback, Nassau, Germany. Stalactites of water-deposited minerals may always be recognized by their rounded outlines.
Psilomelane from Schneeberg, Saxony, Germany. Sometimes there is a tendency to form crystals on the surface of the rounded water-formed masses which gives them a drusy appearance like velvet.
The metallic oxides are represented by a number of widely distributed and important minerals. The copper oxide, cuprite (Case 6), furnishes some handsome groups of isometric crystals of cubic habit and deep red color.
Corundum, the sesquioxide of aluminum, with its richly colored varieties sapphire and ruby, also constitutes a very attractive series. In this instance the crystal forms consist mostly of hexagonal pyramids which are often highly modified.
Hematite (Case 6), the sequioxide of iron, is the principal source of that metal. The series includes a number of varieties, grading from the brilliant crystal groups from Elba and Switzerland to the massive red, loosely compacted material from the ore beds of Michigan. Magnetite is another iron oxide which merits attention because of its importance as a source of iron. Specimens from many of the American and foreign deposits are shown in this series. Cassiterite and rutile are closely related oxides of this group and furnish the visitor with magnificent examples of tetragonal crystals. Many of these are so free from distortion as to be almost diagrammatic in their four-fold symmetry (Compare with models in Case 25). Among the hydrous oxides in Case 8 are two minerals to which attention is particularly directed because of their economic importance as ores and because they illustrate in a striking way the characteristic manner in which this class of minerals has been deposited. Limonite, the hydrated oxide of iron, and psilomelane, the hydrated oxide of manganese, give evidence of their secondary origin by a variety of forms both interesting and curious. Here we find iron oxide which has replaced and taken the crystal forms of other iron minerals, rounded masses deposited layer upon layer, and delicate thread-like stalactites of great beauty.
Carbonates
Cases 8–10 and O–T
Among the simplest of the groups of compounds derived from the oxides of the non-metals are the carbonates, which are combinations of carbon dioxide with one or more of the metallic oxides. At the head of this division stands the mineral calcite, important because of its very wide distribution and its common association with minerals of ore veins, and extremely interesting because of the almost infinite variety of form and habit shown by its crystals. There is no finer example to be found among mineral species of the manifold expression of the law of symmetry in crystallization, which in this instance among thousands of complex manifestations preserves a three-fold symmetry. The series of crystallized calcite in Cases 8 and 9 well illustrates the wide range of forms characteristic of this mineral, from the simple rhombohedra from Poretta and the six-sided prisms from Saxony to the highly complex modifications from Cumberland and Michigan.
Calcite from Cumberland, England. Clear brilliant Crystals with smooth glistening faces.
Calcite from Schneeberg, Saxony, Germany. Plate-like Crystals which are massed in rounded piles resembling sheets of paper.
Dolomite, the carbonate of calcium and magnesium; siderite, the carbonate of iron, and rhodochrosite, the carbonate of manganese, all belong in the same group with calcite and have many of the characteristics of form which were seen in that mineral. They are best distinguished from calcite by the fact that they unite in curved groupings and by their differences of color. The series of siderite (Case 9) and rhodochrosite (Case 10) are especially fine. Aragonite is a second form of calcium carbonate and one which crystallizes in an entirely different way from calcite. Among the suite in Case 10, attention is particularly directed to the branching coral-like forms which distinguish the cave deposits of aragonite and which, with their delicate lacework of fine stalactitic stems, constitute objects of great attractiveness of form.
Aragonite from Eisenerz, Styria. Stalactites of calcium carbonate sometimes form branching forms resembling coral.
Cerussite, the carbonate of lead, is related to aragonite in much the same way that the minerals of the group containing dolomite, siderite, and rhodochrosite are related to calcite; cerussite is mainly formed by the alteration of galena through the action of water charged with carbon dioxide.
The two copper carbonates Malachite and Azurite (Case 10) are attractive by reason of their rich colors and the unique shapes taken by the radiating, silky fibers of the one and the brilliant crystal masses of the other. Like cerussite they are alteration minerals which have resulted from the action of water charged with carbon dioxide on other copper ores.
Malachite from Bisbee, Arizona. The hollows between the rounded masses of this copper carbonate, where water has trickled through, have become crusted with branching riverlets of mineral deposits.
Garnet from Russell, Mass. This mineral is very easily recognized by its isometric crystals.
Natrolite from Leipa, Czechoslovakia. A miniature cavern partly filled with groups of slender crystals.
Silicates
Cases 11– and U–Z
The largest and from some points of view the most important division of the natural chemical compounds which constitute the minerals is that one which has for its basis the combinations of the two commonest elements, silicon and oxygen. The oxygen salts composed of these two elements combined with the oxides of the metals give us the very numerous and varied groups of rock-forming minerals known as the Silicates.
Broadly speaking the silicates are the minerals of the igneous or fire-formed rocks; they are essential constituents of granites, pegmatites, gabbros, diorites and gneisses, and some of them are to be found in crystalline limestones and as secondary minerals lining the cavities of lava, basalt and diabase.
The Feldspars, shown in Case 11, are silicates of aluminum with some other metal. They are the commonest and most widely distributed group of minerals in this division and constitute nearly 60 per cent of the mineral composition of igneous rocks. In the series exhibited, orthoclase, microcline and albite are especially beautiful and interesting, as is also labradorite with its brilliant and varied play of colors.
The Pyroxenes (Case 12) form another important group of silicates embracing a number of closely related minerals, all conforming to a characteristic crystal habit. Here may be seen marked differences of color due to variations in chemical composition as well as differences in transparency from the clear gem-like diopside to the opaque black augite.
Rhodonite (Case 12) is a triclinic pyroxene containing manganese which gives to it a handsome rose color. The specimens of this suite are especially attractive.
The group of Amphiboles (Case 12) constitutes a large and important portion of the silicate division, and like that of the pyroxenes is made up of a number of mineral varieties closely related chemically and based on variations from a standard chemical type.
Cyanite from St. Gotthard, Switzerland. Blade-like crystals in mica schist.
Beryl is a silicate of the rare metal beryllium which furnishes the two well-known precious stones emerald and aquamarine. The many varieties of this mineral are shown in the splendid series to be found in Cases 12 and 13, which furnishes one of the most attractive portions of the collection.
Garnet (Case 13) is a common and widely distributed silicate to be found as an accessory mineral in rocks of almost every kind. Occurring in crystals of a simple and very characteristic isometric habit, garnet displays an amazing range of color in its many varieties. The series exhibited is notably large and complete. Among the important silicates in Case 13 will be found willemite, the silicate of zinc, which occurs in several differently colored varieties at Franklin, N. J.; some magnificent specimens of dioptase, the silicate of copper, in well-formed hexagonal crystals; very attractive suites of wernerite, vesuvianite and zircon (Case 14) in single individuals and groups of tetragonal crystals, and crystallized topaz in a diversity of crystal forms and a variety of color as pleasing to the eye as they are interesting.
Epidote from Tyrol, Austria. A radiated group of brilliant, well formed crystals.
Cases 14 and 15 contain the silicates epidote, prehnite, axinite and the species of tbe humite group, all of which are represented by characteristic series well worth close observation and including among the epidotes and axinites some remarkably fine examples.
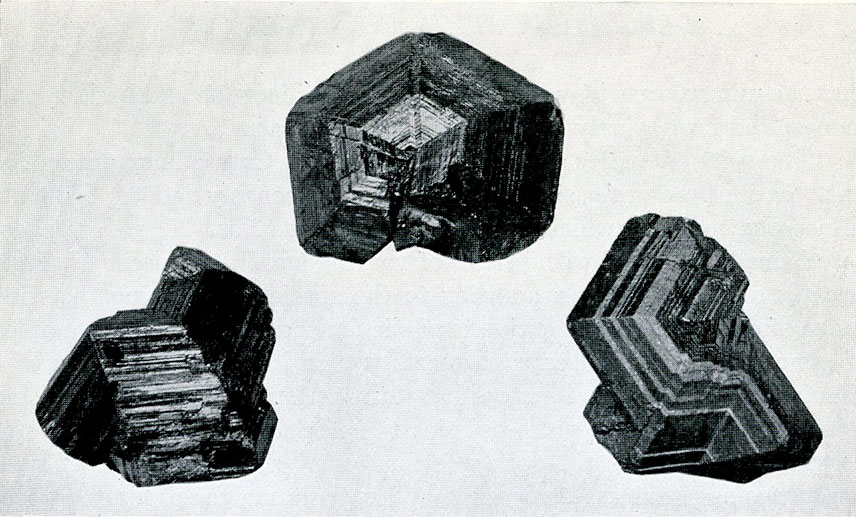
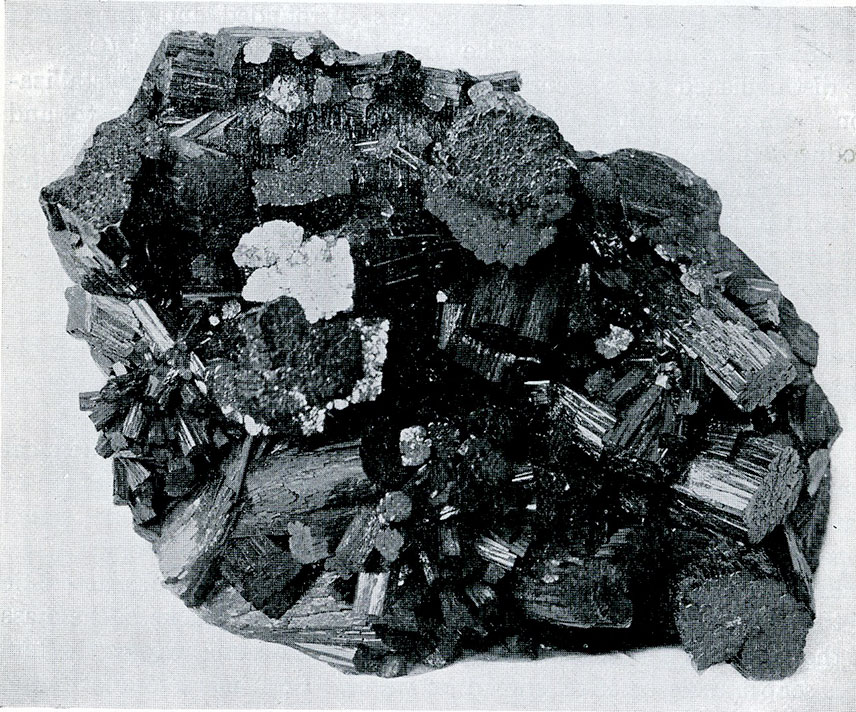
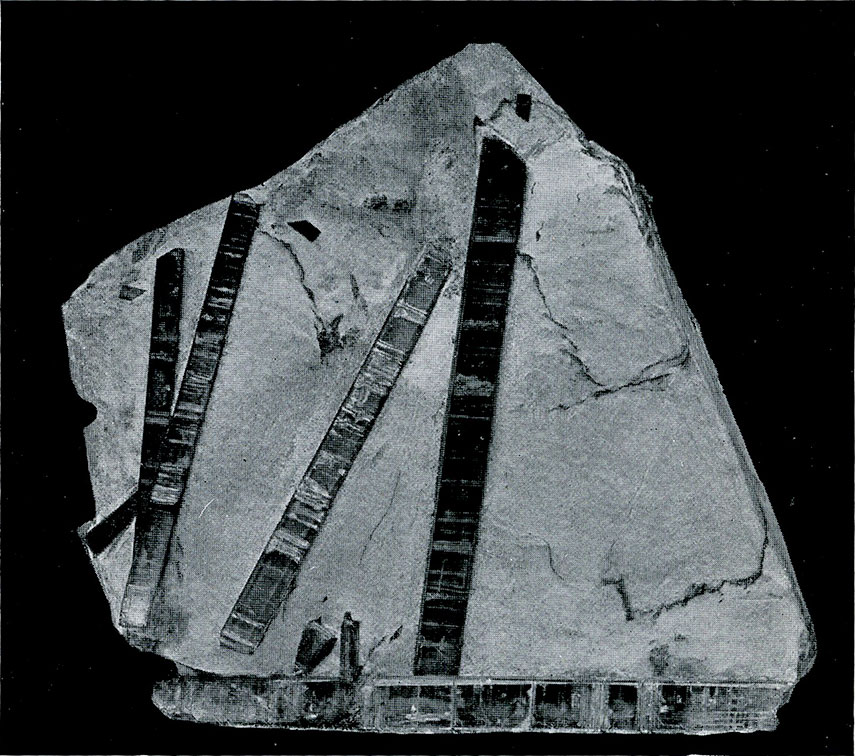
The suite of tourmaline which nearly fills one side of Case 15 is notable for the richness of its display of this very striking mineral. Both in the foreign occurrences and in those from the United States this portion of the collection abounds in beautiful and unusual mounts. Especially interesting are the specimens showing unequal distribution of color from Haddam, Conn., from Pala, Calif., and from Elba, Italy.
The Zeolite Division of the Hydrous Silicates (Cases 15 and 16) includes some large and finely developed tetragonal crystals of apophyllite in single individuals and in large and imposing groups. Here are also to be found the oddly shaped aggregates of heulandite and stilbite, some of which resemble sheaves of wheat, the scattered groupings of chabazite and analcite crystals which often give the appearance of being strewn over a background of dark rock matrix, and the slender, bunched needles of natrolite, springing from a central nucleus like the rays of a sun. The zeolites are essentially minerals of the basaltic or trap rocks and are mostly to be found in cavities, having been deposited in these cup-like hollows by the evaporation of water solutions.
The Mica Division of the Hydrous Silicates, shown in Cases 16 and 17, include minerals which have the distinguishing property of splitting up into thin elastic plates or sheets, as in the familiar example of white mica or isinglass. Many species and subspecies are to be found in the series displayed, the differentiating characters of which are well shown.
The remaining species of the hydrous silicates are contained in Case 17; a few of the first of these, including clinochlore, strongly resemble the micas in general appearance but split into sheets which are not elastic.
Serpentine is a hydrous silicate of this division which has not as yet been found crystallized, although it often takes the crystal forms of other minerals which it has replaced chemically. Many of these replacements or pseudomorphs are to be found in the exhibited series. A fibrous form of serpentine, called chrysotile, is interesting as furnishing much of the asbestos which is woven into fireproof fabric. Talc and sepiolite are also commercially important minerals, the latter furnishing us with the meerschaum from which smoking utensils are made. Two of the hydrous silicates, garnierite, the silicate of nickel, and chryscolla, the silicate of copper, are ores of their respective metals.
The last portion of the large class of the silicates includes a number of mineral species containing both silicon and titanium or in some instances titanium acting alone as an acid, the latter compounds being designated as titanates. Chief among these titano-silicates will be found titanite (Case 17) represented by many varieties, some of which are transparent and gem-like and all of which are interesting to the student and the collector. Although not of economic importance, except as a rather rare gem species, titanite has some interest as an accessory rock-forming mineral. In Case 18 will be found the compounds of the rare elements niobium and tantalum known as columbates and tantalates. These include columbite and samarskite as well as a considerable number of rarer minerals, all of which are very useful as the sources of the group of rare elements such as yttrium, cerium, lanthanium, didyium, etc., which are daily becoming more important commercially.
Phosphates
Cases 18–20 and AA
The Phosphates, which also include the somewhat rarer arsenates, vanadates and antimonates, comprise a considerable and very varied group of minerals. Xenotine and monazite are somewhat related commercially to the columbates and tantalates of the preceding group because they are phosphates of the rare elements previously mentioned. Case 18 contains a very complete series of these. The common mineral apatite (Case 18) is essentially a phosphate of lime and is the most widely distributed of all the phosphates. As is the case with most of the common mineral species, apatite is found in a great many varieties; these differ in color and transparency, but all when crystallized exhibit the characteristic six-sided prism capped by a low pyramid or by a flat base. The large series exhibited shows well the varied difference in form and color of this mineral as well as its almost universal distribution. Pyromorphite and mimetite (Case 18) are respectively the phosphate and arsenate of lead with lead chloride. Both are alteration products occurring in the oxidized portions of lead sulphide deposits, and the commoner of the two, pyromorphite, is ranked as an ore of lead. The brilliant color and unique crystal aggregates to be found in the series of specimens exhibited render these minerals objects of considerable attractiveness.
Vanadinite (Case 19), is the vanadate of lead and bears the same relation to the more commercially important deposits of lead sulphide as do pyromorphite and mimetite. In the series displayed attention is drawn to the beautifully developed hexagonal crystals and the rich and striking colors shown in this handsome suite of specimens. Among the rare phosphates in Case 19 will be found many minerals which by reason of their beauty and interest will well repay a short inspection. Among these may be mentioned descloizite, the lead-zinc vanadate; libethenite and pseudomalachite, the rare copper phosphates; roselite and erythrite, arsenates of cobalt, and variscite, a phosphate of aluminum.
Wavellite (Case 20), another phosphate of aluminum, presents many striking examples of radiating and stalactitic structure combined with colors of choice delicacy and attractiveness. Turquoise, the familiar gem mineral, here takes its place among the phosphates and is represented by a fine series of matrix specimens which illustrates its distribution as well as its slight color variations. The radioactive minerals torbanite, copper uranium phosphate, and autunite, the calcium uranium phosphate, are represented by many specimens, which in the instance of autunite give evidence to the unaided eye, by the singular quality of their yellow green color, of the unusual character of their emanations.
Among the borates in Case 20 will be found a remarkably handsome and complete suite of colemanite, a calcium borate from California. Case 20 also contains the radium minerals uraninite, gummite and carnotite. Of these, uraninite contains the higher percentage of radium, but carnotite, owing to its wider distribution in the Western United States, is becoming the more important radium ore.
Sulphates
Cases 20–21 and BB–CC
Like the phosphates, the minerals of the Sulphate Division are mostly secondary products which have been derived from other minerals or rocks by alteration. The action of water upon most of the sulphides of the metals produces from these sulphuric acid and metallic oxides, which combine to form sulphates. Many of these sulphates are soluble in water and are consequently carried away in solution to be deposited elsewhere, but the larger number of them are to be found in more or less close proximity to the primary minerals from which they were derived.
Barite, the sulphate of barium, is a common and widely distributed mineral species, frequently found in association with metallic ores as a vein mineral. In the series exhibited in Case 20 many examples of the occurrence of barite with sulphides of lead, copper, iron and silver will be found. Like calcite, barite is remarkable for the great diversity and complexity of the crystals in which it forms; splendid specimens of these orthorhombie crystals are shown throughout the suite which is both very complete and of notably high quality. Closely related to barite is the sulphate of strontium, celestite, Although sometimes occurring like barite with metallic ores, celestite is more often found in close association with sulphur and gypsum: an example of the latter association is found in the specimens from Girgenti, Sicily, a magnificent series of which will be found in Case 21. Celestite furnishes the strontium salts which are much used in the manufacture of fireworks, in medicines and in refining sugar.
Barite from Frizington, England. Flat prismatic crystals which show layers of growth.
Anglesite (Case 21) is another sulphate which has an economic importance. This lead mineral is frequently found associated with galena as a decomposition product of the latter and is often mined with it and other ores.
A very striking and beautiful series in Case 21 is that which represents the mineral crocoite, the lead chromate. The bright hyacinth red and orange color, which constitutes one of the chief characteristics of this mineral, is affected by long exposure to the light and consequently the suite of specimens is covered with hinged lids which should be lifted in order to view this exhibit. One of the most common and important of the sulphates is gypsum (Case 21), the hydrous sulphate of calcium. The exhibited suite of specimens is remarkable for the size and quality of its crystallized examples both as single individuals and in large groups. Among the rarer species which are included among the hydrous sulphates are many specimens which combine great beauty of color with interesting structure, features which tend to make this one of the most attractive sections of the collection.
Tungstates, Molybdates
Although represented by very few minerals, this division of the classification contains three important species, wolframite the tungstate of iron and manganese, scheelite the tungstate of calcium, and wulfenite the molybdate of lead. These are all important minerals from a commercial point of view, because they furnish us with the rare metals tungsten and molybdenum which are used to make special steels of a high grade of strength and durability. The series which includes these three, as well as many rarer tungstates and molybdates, will be found in Case 22.
The Minerals of Manhattan Island
Manhattan Island offers the somewhat unique case of a limited area of mineral-producing rocks where excavations have been carried forward to such an extent that practically all of the crystalline rocks which underlie the drift deposits have been exposed at some time, and most of this area has been laid bare within a fairly recent stage in the development of the City. As a consequence of this unusual activity in excavation, much is known concerning the minerals which occur in the local rock formations, and the local collections made from these rock[s] have been both exhaustive and varied. Much of the credit for this intelligent activity in collecting and preserving the local minerals belongs to the members of the New York Mineralogical Club, the results of whose labors in this field may be seen in the Collection of Manhattan Island Minerals, loaned through the courtesy of the New York Mineralogical Club and displayed in Cases 27 and 28. Practically every species of the long list recorded from Manhattan Island is included in this series, which is not only large and representative, but contains many specimens of a quality which renders them noteworthy apart from their unusual local interest. Among these latter are especially fine examples of smoky quartz, chrysoberyl, calcite, orthoclase, oligoclase, albite, beryl, garnet, dumortierite cyanite, tourmaline, stilbite, chabazite, harmotone, muscovite, titanite, xenotime, monazite, etc.
Books
Very few books of a popular nature have been published on the subject of Mineralogy. In addition to the Handbook "The Story of the Minerals" by Herbert P. Whitlock, recently published by the Museum the following books may be read with profit by a beginner in the study of mineralogy.
"Minerals and How to Study Them," by Edward Salisbury Dana. John Wiley and Sons, 1895.
"Popular Guide to Minerals" by L. P. Gratacap. D. Van Nostrand Cornpany, 1912.
"The World's Minerals," by Leonard J. Spencer. Frederick A. Stokes Company, 1916.
"Field Book of Common Rocks and Minerals" by F. B. Loomis. G. P. Putnam's Sons, 1923.
A shelf containing useful books for the student of mineralogy will be found on the study table in the central space opposite the Morgan Memorial Tablet, and will be made accessible to any one desiring to consult them.
Although there is much to be gained by the student of mineralogy from books, and although they furnish a very necessary key to the meaning of what is to be seen in the mineral world, the best and most satisfactory knowledge of the subject is to be gained from studying collections of minerals. The knowledge which enables one to recognize a mineral at sight is similar to the knowledge which enables one to recognize a friend. It is a composite realization of a number of characteristics, no one of which is sufficiently definite and unique to be relied on without the aid of some of the others. We may read a statement of the form, the color, the luster and the various other attributes of a certain mineral, but until we have these combined properties set before our eyes in a specimen of that mineral we can form only an imperfect idea of it.